Which Statement Best Describes The Properties Of Covalent Compounds?
Which statement best describes the properties of covalent compounds?. The module presents chemical bonding on a sliding scale from pure covalent to pure ionic depending on differences in the electronegativity of the bonding atoms. 51 kilometers Asked By adminstaff 20012020 0315 AM. 1 Answers Which statement best describes the properties of covalent compounds.
Properties of ionic and covalent compounds DRAFT. Which statement best describes the properties of ionic compounds. Properties of Covalent Compounds Most covalent compounds have relatively low melting points and boiling points.
The millions of different chemical compounds that make up everything on Earth are composed of 118 elements that bond together in different ways. When dissolved in water they dont conduct electricity. Covalent compounds tend to be more flammable than ionic compounds.
Which statement best describes the properties of metals. Which statement best describes the atoms of elements that form compounds by covalent bonding. The bonds of metallic substances are composed.
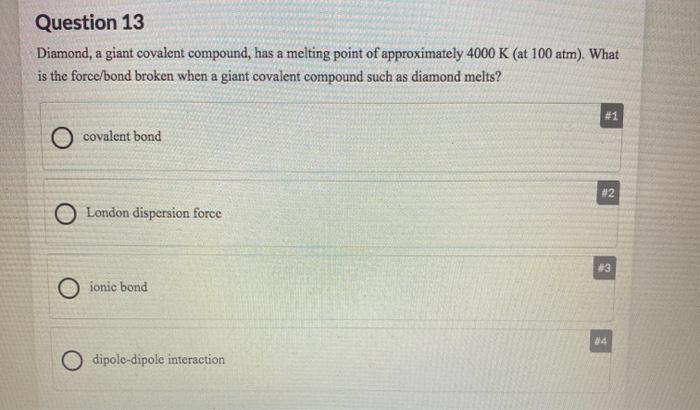
They are brittle solid at room temperature and have a high melting point. The picture shows his lab notebook. A covalent compound because it has a low melting point.
They mostly have low melting and boiling points. You can recognize covalent compounds because they consist only of nonmetals. Click again to see term 115.
They are good conductors of heat and electricity and are ductile and malleable. Volatile c Conduct electricity in the molten state or in an aqueous solution but do not conduct electricity in the solid state.
The millions of different chemical compounds that make up everything on Earth are composed of 118 elements that bond together in different ways.
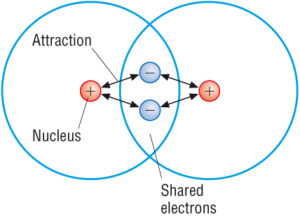
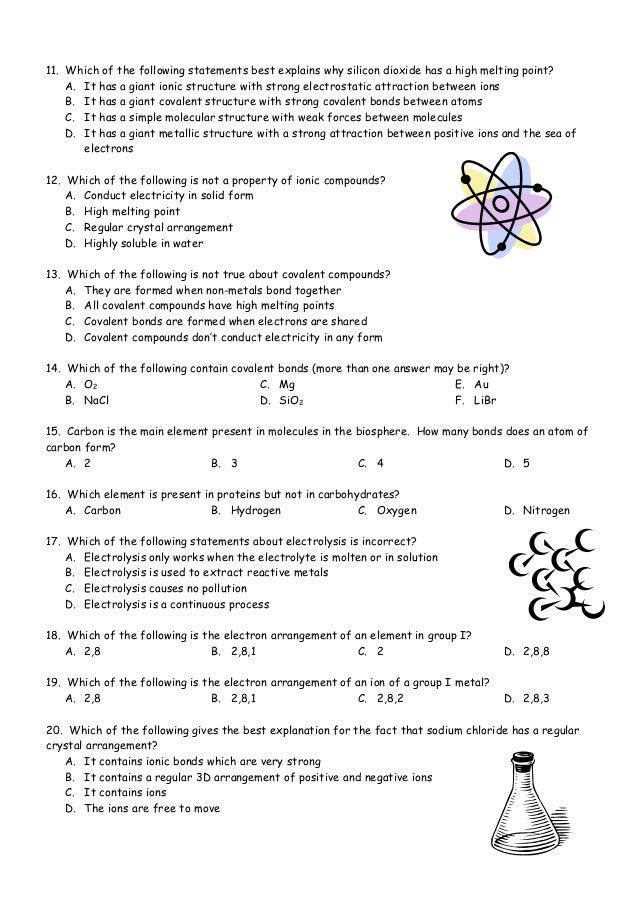
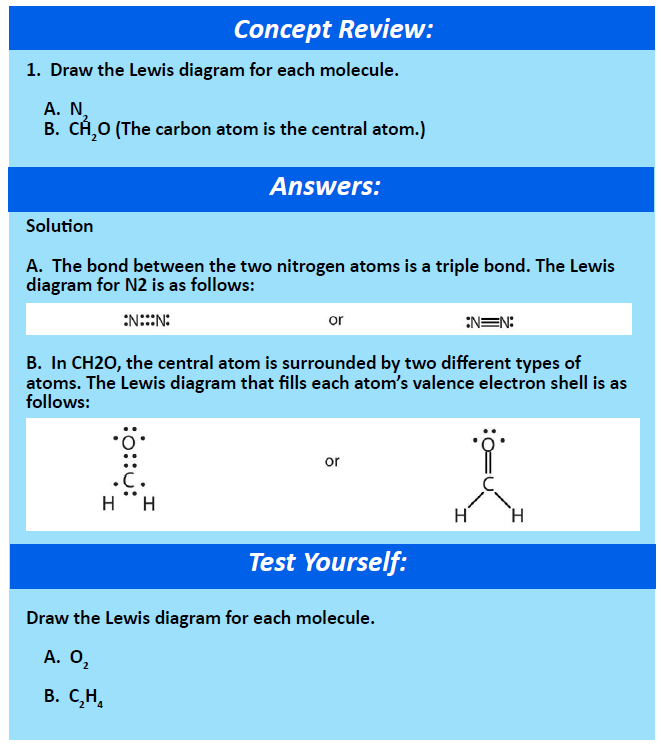
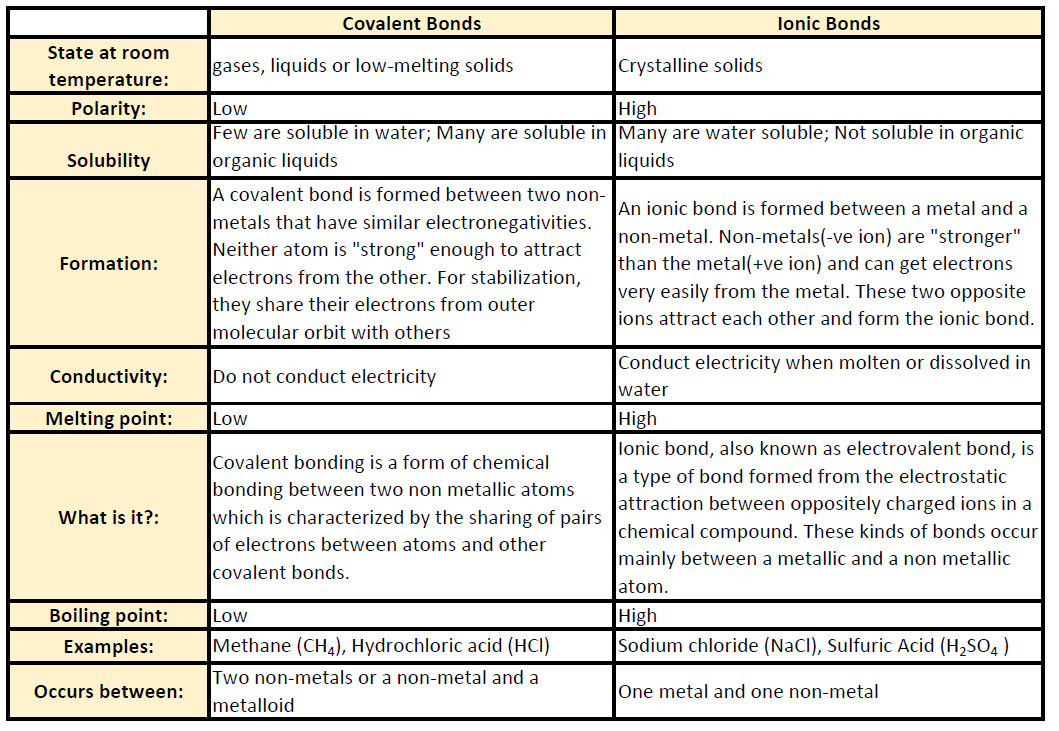
You can recognize covalent compounds because they consist only of nonmetals. LiCl and C6H14O have different atomic masses. This is because covalent compounds dissolve into molecules while ionic compounds dissolve into ions which can conduct charge. Covalent compounds Ionic compounds composed of simple molecules a Have high melting and boiling points a Have low melting and boiling points b Exist as solids at room temperature. Which statement correctly describes the behavior of the valence electron in the ionic bond. Properties of Covalent Compounds Most covalent compounds have relatively low melting points and boiling points. Non-volatile b Usually exist as liquids or gases at room temperature. Which statement best describes the properties of ionic compounds. The bonds of metallic substances are composed.
Ionic bonds could be best described as. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. They are always solid at room temperature are brittle and have a high melting point. Ionic compounds conduct electricity when dissolved in water while covalent compounds typically dont. One compound is ionic and the other is metallic. Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds Covalent compounds tend to be soft and relatively flexible. Which statement best describes the mistake he.
















/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)








/diamond-680790317-5b368650c9e77c0037c34182.jpg)

/artwork-of-water-molecules-581746593-595a8e8f3df78c4eb6cbec33.jpg)










Post a Comment for "Which Statement Best Describes The Properties Of Covalent Compounds?"